Lead asset
INHAL-101
THE MOLECULE
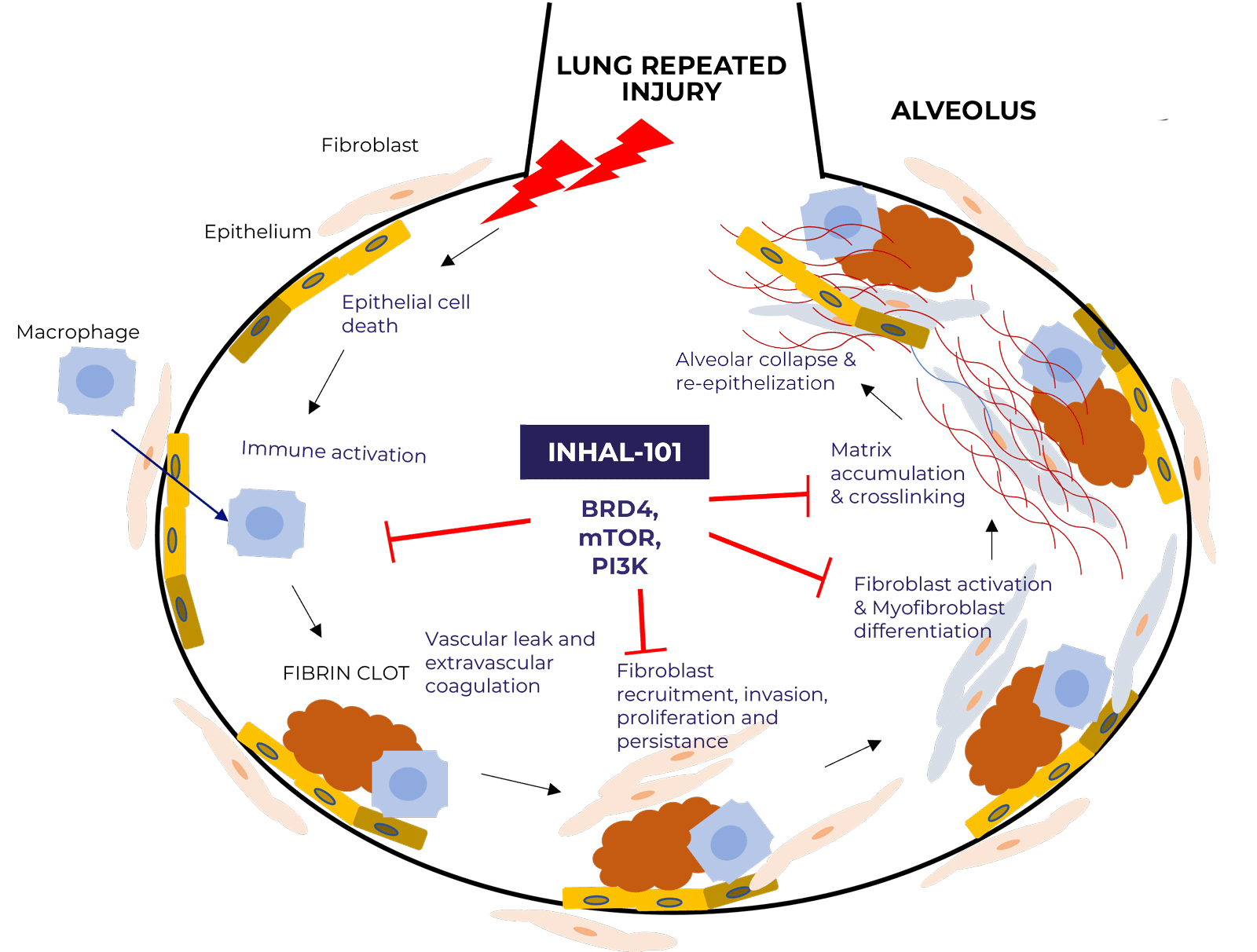
INHAL-101 has a unique MoA targeting three main pathways known to be key underlying causes of IPF, including the BRD4 target that is an important epigenetic modulator in pulmonary fibrosis. It showed a very selective binding profile, tested on high-throughput panels of targets including a 232 human kinase panel.
IPF pathogenesis and mOA of INHAL-101
PI3K
mTOR
BRD4
- Growth factor signaling (including TGFβ)
- Epithelial Mesenchymal Transition
- Conversion of fibroblasts to activated myofibroblasts
- Deposition of fibrotic extracellular matrix
Parental administration of INHAL-101 has demonstrated an excellent safety profile in all animal studies even up to 3 times the efficacy dosage. This systemic administration safety profile offers a comfortable safety window for administration through pulmonary inhalation.
In animal testing INHAL-101 was shown to be highly effective in preventing fibrosis development and disease progression with remarkably improved survival of 80% versus only 29% survival for the untreated group.
Thanks to its Mechanism of Acton, iINHAL-101 has the potentiality for stopping and even reversing fibrosis in certain cases.
The inhaled form, formulated with Industry leading mSAS ® technology, is delivered to deep lung up to 4X more than other technologies. Extensive histopathology studies of the entire respiratory tract, even at high doses, demonstrate no signs of irritation representing excellent tolerability and minimal risk of cough reflex. These studies confirm an important advantage in terms of treatment compliance in IPF patients. From pharmacokinetic studies only 1/3 of the inhaled dose passes out of the lung into the bloodstream where it is cleared very quickly thus minimizing any potential systemic side effects. Favorable high retention time in rat lung tissues as well as favorable kinetics were observed for inhaled INHAL-101.
mSAS® TECHNOLOGY
mSAS® is a scalable platform to generate stable high-performing particles in a 1-step process. The process achieves industry-leading particle delivery performance to reach the deep lung up to 4X more than other technologies showing excellent inhalation performance even at low inspiratory pressures characteristic of patients with respiratory impairment.
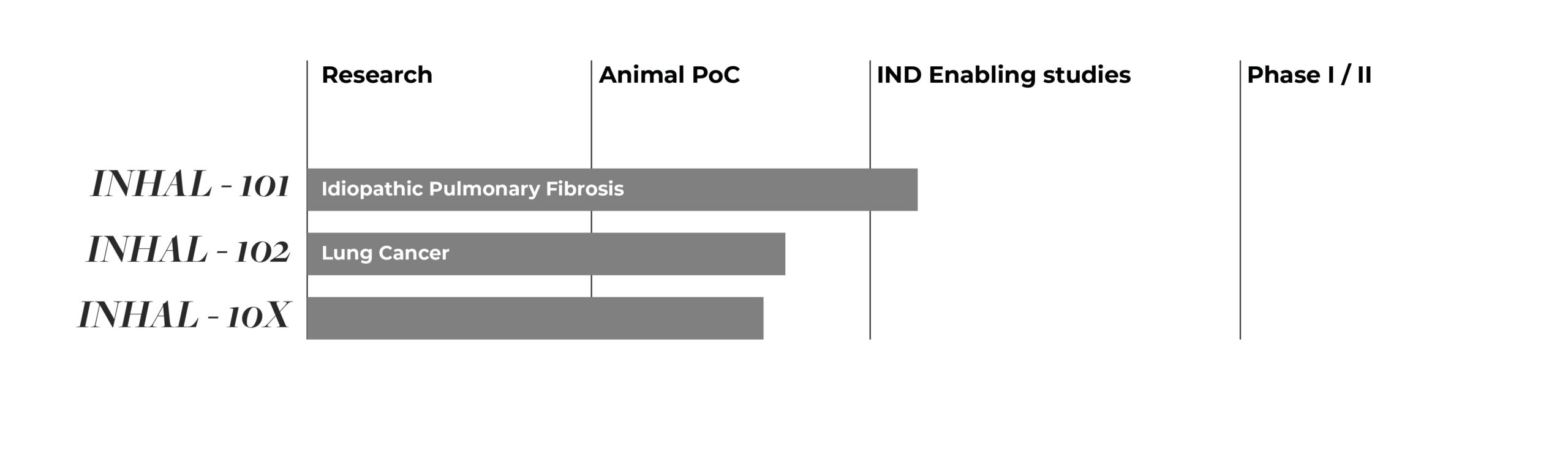
pipeline
Preclinical Proof-of-Concept obtained in several disease models including cancer and other fibrotic diseases along with pulmonary infections.
©2022 INHALIS
All rights reserved.
Policy / Credits